So realized I didn’t really understand how water boils. I used to think it was because the higher the temperature, the larger the fraction of molecules that had enough energy to break free.
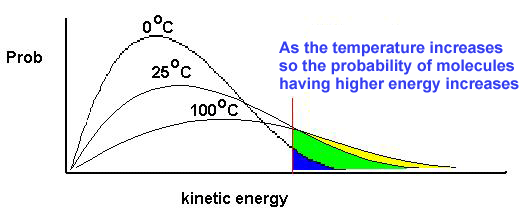
That is true, but it means that the evaporation rate gradually increases as temperature rises. The speed wouldn’t suddenly spike at the boiling point. It also doesn’t explain why it isn’t possible to heat water above the boiling point, or why the boiling point is dependent on pressure.
The answer is bubbles. At the boiling point, most molecules are actually still too slow to vaporize. Instead what happens is the equilibrium vapour pressure becomes greater than the atmospheric pressure, allowing bubbles of vapour to form underwater. At this point, molecules anywhere inside the water with enough energy can vaporize, not just the few layers at the surface. So the vaporization rate dramatically increases until it is fast enough to absorb whatever energy is being input.
So what happens when the average energy actually approaches the activation energy? The evaporation rate has become very high even without bubbles, so the water will vaporize quickly regardless of atmospheric pressure, causing the vapour pressure to increase until it is as dense as the water. Now it is nearing the critical point.